We're seeking patients for a phase 3 clinical research study
Have you been diagnosed with multiple system atrophy (MSA) and have dizziness symptoms associated with neurogenic orthostatic hypotension (nOH)? If so, you may qualify.
We are researching the investigational drug (ampreloxetine) because we believe it may reduce nOH symptoms that interfere with daily activity in MSA patients based on prior clinical trial results. We are running the Phase 3 CYPRESS trial to confirm whether the investigational drug is effective and safe in treating nOH symptoms in MSA patients and to learn about any unwanted side effects.
STUDY HIGHLIGHTS
Duration of study
Study visits
Ampreloxetine is not currently approved by any health agency for use in treating symptoms of nOH in MSA patients.

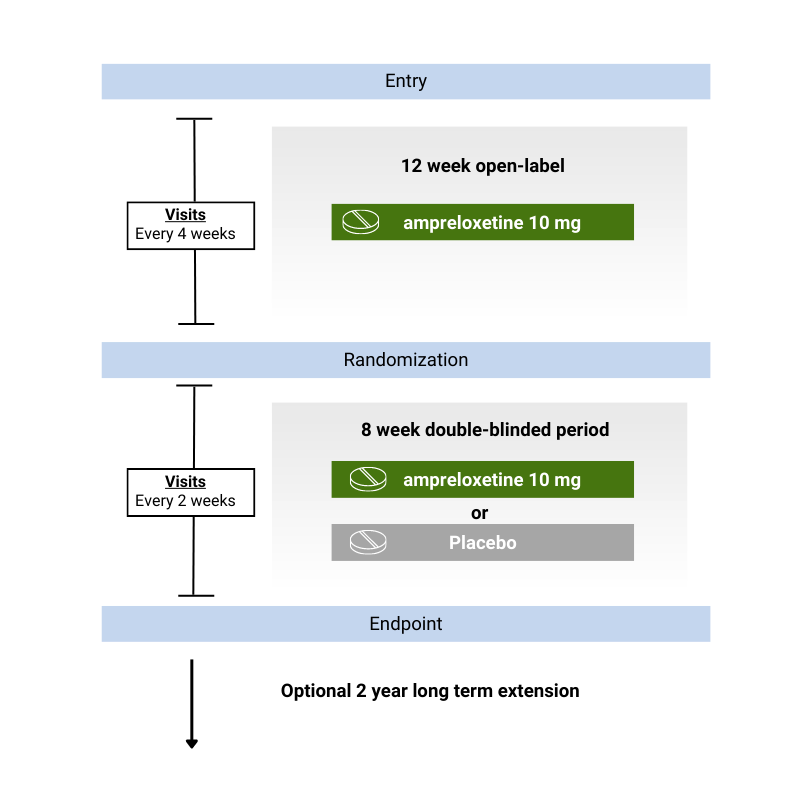

"Open-label" means both the health providers and the patients are aware of the drug or treatment being given.
"Double-blinded" means that neither you nor your study doctor will know if you are taking the study drug or placebo.

You may be eligible to take part in the CYPRESS Study if you:
You will also need to meet additional criteria to qualify. A study doctor will explain these.
If someone has symptomatic nOH, it means they are experiencing symptoms of nOH, such as:
We know how important it is to make clinical trial participation as easy as possible. We built flexibility into our protocol and logistics, enabling you to potentially participate by either:
Enrolling at one of our open clinical sites
OR
Referring us to your doctor of choice
If referring us to your doctor of choice, we will approach them to determine their interest and ability to participate in the study.
For additional flexibility, our study allows all visits other than the screening visit to be conducted in the comfort of your home.
Please contact us directly to explore how we might address your specific situation. We'd love to hear from you!
For More Information
To learn more about the CYPRESS Study, please go to www.clinicaltrials.gov, email us at cypress@theravance.com, or call us +1-855-633-8479 for more information

For more information: clinicaltrials.gov
A Phase 3 clinical research study (also called a clinical trial) is a medical study that helps to answer important questions about an investigational drug or device, such as: Does the investigational drug work or how effective is it compared to other drugs/devices?
This research study is testing an investigational drug (ampreloxetine) compared to placebo in people with MSA that experience symptomatic nOH. It will help answer the following questions:
You can talk to a study doctor to find out if you may qualify for the study. Contact us at cypress@theravance.com or by calling +1-855-633-8479 and we can see if there is a study site near you.
There is no cost to participate in the CYPRESS Study. The investigational drug, study-related tests, assessments, and study visits will be provided at no cost to you and your insurance company. If you decide to take part in the study, you will receive study-related care throughout the study from a team of experienced doctors and nurses.
No health insurance is required to participate.
Volunteers who take part in the study may be compensated for travel costs and time in the study. Please discuss this with the study site when you are in contact with them.
The research team at your study site will be able to explain more about what the CYPRESS Study will involve, and it is up to you to decide if you want to participate. Participation in this study is voluntary. Whether or not you decide to participate in this study will not affect your current or future relationships with your doctors. If you decide to participate, you are free to withdraw at any time without affecting those relationships.
There are risks and benefits of taking part in any clinical study. For example, your health may be more closely monitored than it would have been otherwise. You may or may not benefit from the investigational drug (ampreloxetine). It is not known if your condition will get any better; it may get worse. This study is being conducted to learn more about the investigational drug. What we learn may help other MSA patients in the future. The known risks and benefits of participation are outlined in the informed consent form (ICF) that you must read and sign before you can take part in this study. You should discuss the risks and benefits of participating with your doctor.
Please contact us to explore further options, including potential travel support.
You will need to attend the site/research center (in-clinic) for your screening visit as part of the study. For the remaining study visits, you and your study doctor can discuss completing those study visits either in the site/research center (in-clinic), or remotely (e.g. your home or residence).
Participants in the CYPRESS study are restricted from participating in other studies in which they receive an investigational drug. Participation in an observational study that does not require interventional treatment (e.g. another study drug from a different trial) is permitted.
The Investigational drug (ampreloxetine) was assessed in a study that had a similar design to the CYPRESS study. In that study, in a pre-specified population of MSA participants, there was a durable and clinically meaningful improvement in the symptoms of nOH in participants with MSA. The results of this study are available on the Theravance Biopharma website. We are running the Phase 3 CYPRESS trial to confirm the results of the prior trail.